The Huanan seafood and wildlife market in China has been a focal point in the search for the origin of the coronavirus that has impacted the world as none other in the past three years. People around the world have been seeking to identify the origin of the deadly virus.
Now a research team in China has published analysis of samples taken more than three years ago from the market linked to the outbreak of Covid-19. This is the first peer-reviewed study of biological evidence gathered from the market back in 2020.
By linking the virus with animals sold in the market, it could open new lines of inquiry into how the outbreak began. The research reveals swabs that tested positive for the virus also contained genetic material from wild animals.
Some scientists say this is further evidence that the disease was initially transmitted from an infected animal to a human. But others have urged caution in interpreting the findings and it remains unclear why it took three years for the genetic content of the samples to be made public.
Another theory has centered on the suggestion that the virus accidentally leaked from a laboratory in Wuhan.
No definitive proof
The Chinese research team posted an early version of their study online back in February 2022, but they did not publish the full genetic information that was contained in the samples gathered from the market.
In March this year, another international group of researchers shared their own assessment of what those crucial market swabs had revealed, after spotting that the genetic sequences had been posted on a scientific data-sharing website.
This new analysis, which has been validated by other scientists before being published in the journal Nature, includes more important detail about the content of those samples, which were collected from stalls, surfaces, cages and machinery inside the market. Before the 2020 outbreak, scientists took photos of animals, including racoon dogs, being sold in the Huanan market
The Chinese research team’s paper showed that some samples – collected from areas where wildlife was being sold – had tested positive for the virus. Their analysis also showed that animals now known to be susceptible to the virus, particularly raccoon dogs, were being sold alive in those locations.
But the Chinese researchers have pointed out that their discoveries fall short of definitive proof of how the outbreak started. “These environmental samples cannot prove that the animals were infected,” the paper explains. The possibility remains, it adds, that the virus was brought into the market by an infected person, rather than an animal.
Prof David Robertson, from the University of Glasgow, is a virologist who has been involved in the genetic investigation into the origin of SARS-CoV-2 since it emerged in 2020. He told BBC News: “The most important thing is that this very important dataset is now published and available for others to work on.”
But he added that the contents of the samples were “compelling evidence that animals there were probably infected with the virus. It’s the whole body of evidence that’s important,” he said. “When you bring this together with the fact that the early Covid-19 cases in Wuhan are linked to the market, it’s strong evidence that this is where a spillover from an animal in the market occurred.”
According to BBC, the published findings come amid signs that the lab leak theory is gaining ground among authorities in the US. The Chinese government has strenuously denied suggestions that the virus originated in a scientific facility, but the FBI said it now believes that scenario is the “most likely”, as does the US Department of Energy.
Various US departments and agencies have investigated the mystery and produced differing conclusions, but on March 1st, the FBI’s director accused Beijing of “doing its best to try to thwart and obfuscate”, and disclosed the bureau had been convinced of the lab leak theory “for quite some time now”.
The FBI has not made their findings public, which has frustrated some scientists. The lead researcher of the new report, from the Chinese Center for Disease Control and Prevention (China CDC) in Beijing, has been contacted by the BBC for comment.











 Wang is something of a rarity in the world of battery engineering—he’s been in the field since the early 1990s, contributing work on GM’s groundbreaking EV1, and his papers stretching back through the decades have been referenced by hundreds of other studies. About seven years ago, Wang and his team started looking into the question of how to make batteries charge faster. They tried several approaches, including methods to modulate the electrical current feeding energy into the battery, but ultimately cast that option aside.
Wang is something of a rarity in the world of battery engineering—he’s been in the field since the early 1990s, contributing work on GM’s groundbreaking EV1, and his papers stretching back through the decades have been referenced by hundreds of other studies. About seven years ago, Wang and his team started looking into the question of how to make batteries charge faster. They tried several approaches, including methods to modulate the electrical current feeding energy into the battery, but ultimately cast that option aside. 

 Called Vuity, the drop is applied to each eye once a day and starts working within 15 minutes of application. The makers say each drop lasts for at least six hours. As per the report, the drug is a formulation of a well-known compound known as pilocarpine.
Called Vuity, the drop is applied to each eye once a day and starts working within 15 minutes of application. The makers say each drop lasts for at least six hours. As per the report, the drug is a formulation of a well-known compound known as pilocarpine. Dr. Hashima Hasan,
Dr. Hashima Hasan,  Further debut pictures from James Webb are due to be released by Nasa in a global presentation on Tuesday.
Further debut pictures from James Webb are due to be released by Nasa in a global presentation on Tuesday. “Our results tell a tale of two hemispheres. The north has seen major reductions in river sediment transport over the past 40 years, while the south has seen large increases over the same period,” says lead author Evan Dethier, a post-doctoral fellow at Dartmouth. “Humans have been able to alter the world’s biggest rivers at rates that are unprecedented in the recent geologic record.” Dethier says. “The amount of sediment rivers carry is generally dictated by natural processes in watersheds, like how much rain there is or whether there are landslides or vegetation. We find that direct human activities are overwhelming these natural processes, and even outweighing the effects of climate change.”
“Our results tell a tale of two hemispheres. The north has seen major reductions in river sediment transport over the past 40 years, while the south has seen large increases over the same period,” says lead author Evan Dethier, a post-doctoral fellow at Dartmouth. “Humans have been able to alter the world’s biggest rivers at rates that are unprecedented in the recent geologic record.” Dethier says. “The amount of sediment rivers carry is generally dictated by natural processes in watersheds, like how much rain there is or whether there are landslides or vegetation. We find that direct human activities are overwhelming these natural processes, and even outweighing the effects of climate change.” About three-quarters of all Covid-19 deaths have been among seniors, including more than a quarter among people 85 and older, according to CDC data. And while racial and ethnic disparities have lessened over the course of the pandemic, the risk of dying from Covid-19 has been about two times higher for Blacks, Hispanics and American Indians compared to Whites in the US.
About three-quarters of all Covid-19 deaths have been among seniors, including more than a quarter among people 85 and older, according to CDC data. And while racial and ethnic disparities have lessened over the course of the pandemic, the risk of dying from Covid-19 has been about two times higher for Blacks, Hispanics and American Indians compared to Whites in the US. Einstein’s theory works well in explaining the way matter behaves on a large scale but clashes with the way quantum physics describes the gravity among the smallest observable particles. For years, Gionti has attempted to reconcile the two.
Einstein’s theory works well in explaining the way matter behaves on a large scale but clashes with the way quantum physics describes the gravity among the smallest observable particles. For years, Gionti has attempted to reconcile the two. In 2017, SCORE2 clinical trial investigators reported that two types of anti-VEGF treatment were equally effective at improving visual acuity in people with macular edema due to CRVO or hemi-retinal vein occlusion (HRVO). CRVO affects the entire retina, while HRVO generally affects about half of the retina. Half of the study participants had been given Avastin (bevacizumab) while the other half received Eylea (aflibercept). Both drugs were administered by injection once per month for six months. At the six-month mark, the vision of participants in both groups had, on average, improved over three lines on an eye chart.
In 2017, SCORE2 clinical trial investigators reported that two types of anti-VEGF treatment were equally effective at improving visual acuity in people with macular edema due to CRVO or hemi-retinal vein occlusion (HRVO). CRVO affects the entire retina, while HRVO generally affects about half of the retina. Half of the study participants had been given Avastin (bevacizumab) while the other half received Eylea (aflibercept). Both drugs were administered by injection once per month for six months. At the six-month mark, the vision of participants in both groups had, on average, improved over three lines on an eye chart. “Today’s authorization is yet another example of the rapid innovation occurring with diagnostic tests for COVID-19,” Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Today’s authorization is yet another example of the rapid innovation occurring with diagnostic tests for COVID-19,” Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, said in a statement. “Despite their widespread use, traditional medicine lack robust evidence, data and a standard framework preventing their integration into the mainstream healthcare delivery system,” Dr Singh said in an exclusive interview with ANI.
“Despite their widespread use, traditional medicine lack robust evidence, data and a standard framework preventing their integration into the mainstream healthcare delivery system,” Dr Singh said in an exclusive interview with ANI. “It’s really, really, really hard to walk people back from that ledge,” Gill said. Doomism “is definitely a thing,” said Wooster College psychology professor Susan Clayton, who studies climate change anxiety and spoke at a conference in Norway last week that addressed the issue. “It’s a way of saying ‘I don’t have to go to the effort of making changes because there’s nothing I can do anyway.’”
“It’s really, really, really hard to walk people back from that ledge,” Gill said. Doomism “is definitely a thing,” said Wooster College psychology professor Susan Clayton, who studies climate change anxiety and spoke at a conference in Norway last week that addressed the issue. “It’s a way of saying ‘I don’t have to go to the effort of making changes because there’s nothing I can do anyway.’” In addition to the protection offered by vaccination, many people across the country were infected during the first omicron wave over the winter, which means much of the population still has additional immunity.
In addition to the protection offered by vaccination, many people across the country were infected during the first omicron wave over the winter, which means much of the population still has additional immunity.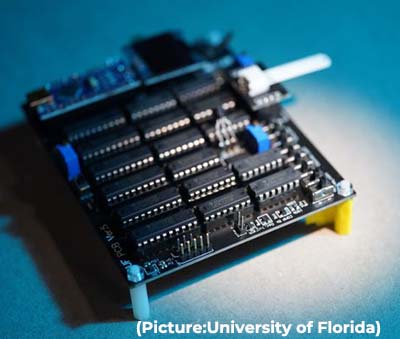 UF has entered into a licensing agreement with a New Jersey company, Houndstoothe Analytics, in hopes of ultimately manufacturing and selling the device, not just to medical professionals but also to consumers.
UF has entered into a licensing agreement with a New Jersey company, Houndstoothe Analytics, in hopes of ultimately manufacturing and selling the device, not just to medical professionals but also to consumers. With a brief exception in the summer of 2021, Covid-19 has consistently been one of the top three causes of death for the past two years in the US, an
With a brief exception in the summer of 2021, Covid-19 has consistently been one of the top three causes of death for the past two years in the US, an 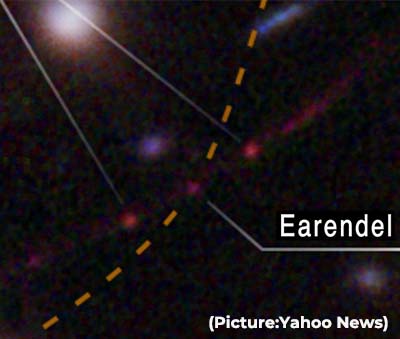 “We almost didn’t believe it at first, it was so much farther than the previous most-distant, highest redshift star,” said astronomer Brian Welch of the Johns Hopkins University in Baltimore, lead author of the
“We almost didn’t believe it at first, it was so much farther than the previous most-distant, highest redshift star,” said astronomer Brian Welch of the Johns Hopkins University in Baltimore, lead author of the  Dr Peter Hotez, Dean of the National School of Tropical Medicine at Baylor College of Medicine (BCM) in Houston during a recent webinar said that the two mRNA vaccines may not impact the world’s low- and middle-income countries, but India’s vaccines, made in collaboration with universities across the world such as BCM and the Oxford University, have “rescued the world” and its contributions must not be underestimated.
Dr Peter Hotez, Dean of the National School of Tropical Medicine at Baylor College of Medicine (BCM) in Houston during a recent webinar said that the two mRNA vaccines may not impact the world’s low- and middle-income countries, but India’s vaccines, made in collaboration with universities across the world such as BCM and the Oxford University, have “rescued the world” and its contributions must not be underestimated. “Why would sleeping with your lights on affect your metabolism? Could that explain why there is a higher prevalence of diabetes or obesity (in society)?” Zee asked.
“Why would sleeping with your lights on affect your metabolism? Could that explain why there is a higher prevalence of diabetes or obesity (in society)?” Zee asked. But if you step inside these campuses and peer into the minds of the people who work, teach and learn here, there is something that is distinctly common running through all of them. It is a set of values, beliefs, and practices, all at once.
But if you step inside these campuses and peer into the minds of the people who work, teach and learn here, there is something that is distinctly common running through all of them. It is a set of values, beliefs, and practices, all at once. The Wireless Sensor Network for Landslide Detection, for example, is a low-cost system that integrates knowledge from multiple domains earth science, communication & networking, analog and digital circuits, to name a few. The system has been designed to detect landslides twenty-four hours ahead of time. It has received a U.S patent, and the university, as a result, is now officially recognized as a World Centre of Excellence for Disaster Risk Reduction.
The Wireless Sensor Network for Landslide Detection, for example, is a low-cost system that integrates knowledge from multiple domains earth science, communication & networking, analog and digital circuits, to name a few. The system has been designed to detect landslides twenty-four hours ahead of time. It has received a U.S patent, and the university, as a result, is now officially recognized as a World Centre of Excellence for Disaster Risk Reduction.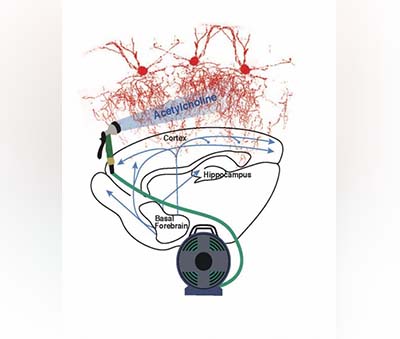 Previous post-mortem studies have shown that the synaptic terminals located at the end of chandelier cell axons appear to be reduced in the brains of patients with schizophrenia.
Previous post-mortem studies have shown that the synaptic terminals located at the end of chandelier cell axons appear to be reduced in the brains of patients with schizophrenia. “We were surprised to find this potential therapy shuts down a novel pathway for viral replication and also protects infected cells,” said
“We were surprised to find this potential therapy shuts down a novel pathway for viral replication and also protects infected cells,” said  Dr. Anupam Sibal, President of GAPIO and Group Medical Director, Apollo Hospitals Group and Senior Consultant Pediatric Gastroenterologist and Hepatologist said, “The awardees through their immense contribution in clinical care, academics, research in different medical and surgical specialities exemplify the highest standards that Indian physicians have become synonyms with.”
Dr. Anupam Sibal, President of GAPIO and Group Medical Director, Apollo Hospitals Group and Senior Consultant Pediatric Gastroenterologist and Hepatologist said, “The awardees through their immense contribution in clinical care, academics, research in different medical and surgical specialities exemplify the highest standards that Indian physicians have become synonyms with.” We are concerned that the Biden Administration is not taking preparedness seriously enough. It was a welcome step to see the Administration
We are concerned that the Biden Administration is not taking preparedness seriously enough. It was a welcome step to see the Administration  The committee, headed by IIT Council Standing Committee Chairman Dr K Radhakrishnan, has been asked to “submit a framework/structure for the opening of campuses abroad by Higher Education Institutes after examining the existing provisions for the opening of offshore campuses” by March 17.
The committee, headed by IIT Council Standing Committee Chairman Dr K Radhakrishnan, has been asked to “submit a framework/structure for the opening of campuses abroad by Higher Education Institutes after examining the existing provisions for the opening of offshore campuses” by March 17. In adults, small amounts can quickly cause dangerously low blood pressure, dizziness, fainting, or even heart attacks or strokes, said Dr. Kelly Johnson-Arbor, Co-Medical Director of the National Capital Poison Center in Washington, D.C. Higher doses can be fatal, she and her colleagues wrote in The American Journal of Emergency Medicine. Sodium azide levels in COVID-19 rapid test kits are not always high enough to cause low blood pressure in adults, and the iHealth kits being sent out by the U.S. government do not contain any sodium azide at all, Johnson-Arbor said. “However… since children are typically much smaller than adults, they are at a higher risk of experiencing poisonous effects after swallowing any amount,” she said.
In adults, small amounts can quickly cause dangerously low blood pressure, dizziness, fainting, or even heart attacks or strokes, said Dr. Kelly Johnson-Arbor, Co-Medical Director of the National Capital Poison Center in Washington, D.C. Higher doses can be fatal, she and her colleagues wrote in The American Journal of Emergency Medicine. Sodium azide levels in COVID-19 rapid test kits are not always high enough to cause low blood pressure in adults, and the iHealth kits being sent out by the U.S. government do not contain any sodium azide at all, Johnson-Arbor said. “However… since children are typically much smaller than adults, they are at a higher risk of experiencing poisonous effects after swallowing any amount,” she said. “Our COVID-19 vaccine has been administered to hundreds of millions of people around the world, protecting people from COVID-19 infection, hospitalization and death,” Moderna CEO Stéphane Bancel said in a statement. “The totality of real-world data and the full [approval] for Spikevax in the United States reaffirms the importance of vaccination against this virus. This is a momentous milestone in Moderna’s history as it is our first product to achieve licensure in the U.S.”
“Our COVID-19 vaccine has been administered to hundreds of millions of people around the world, protecting people from COVID-19 infection, hospitalization and death,” Moderna CEO Stéphane Bancel said in a statement. “The totality of real-world data and the full [approval] for Spikevax in the United States reaffirms the importance of vaccination against this virus. This is a momentous milestone in Moderna’s history as it is our first product to achieve licensure in the U.S.” “We see space as an area for our once-in-a-century transformation. By going to space, we may be able to develop telecommunications and other technology that will prove valuable to human life,” Sato told The Associated Press.
“We see space as an area for our once-in-a-century transformation. By going to space, we may be able to develop telecommunications and other technology that will prove valuable to human life,” Sato told The Associated Press. “We anticipate that there will be a period of quiet before Covid-19 may come back towards the end of the year, but not necessarily the pandemic coming back,” Kluge said. Top US scientist Anthony Fauci expressed similar optimism on Sunday.
“We anticipate that there will be a period of quiet before Covid-19 may come back towards the end of the year, but not necessarily the pandemic coming back,” Kluge said. Top US scientist Anthony Fauci expressed similar optimism on Sunday. The worst-case scenario is the emergence of a still-more dangerous variant, he said. He said this possibility is more reason for people to get vaccinated and receive booster shots, and to make testing and medical treatment more widely available.
The worst-case scenario is the emergence of a still-more dangerous variant, he said. He said this possibility is more reason for people to get vaccinated and receive booster shots, and to make testing and medical treatment more widely available. Thirteen rounds were conducted on the DD National channel between 7 and 7:30 am. Many leading Yoga gurus and institutes like the Indian Yoga Association, National Yoga Sports Federation participated. In his address, Sonowal highlighted the advantages, “Scientifically, the Surya Namaskar has been known to develop immunity and improve vitality, which is significant to our health during the pandemic conditions.”
Thirteen rounds were conducted on the DD National channel between 7 and 7:30 am. Many leading Yoga gurus and institutes like the Indian Yoga Association, National Yoga Sports Federation participated. In his address, Sonowal highlighted the advantages, “Scientifically, the Surya Namaskar has been known to develop immunity and improve vitality, which is significant to our health during the pandemic conditions.” “The study sought to quantify the frequency of and risk factors for severe outcomes in children with COVID-19,” says study co-lead
“The study sought to quantify the frequency of and risk factors for severe outcomes in children with COVID-19,” says study co-lead  We have learned that our Milky Way is one of 2 trillion known galaxies in the universe. Our galaxy alone has 200 billion stars and most of these stars appear to have planets. The probability of intelligent life somewhere in our galaxy or in another galaxy is high, given these numbers.
We have learned that our Milky Way is one of 2 trillion known galaxies in the universe. Our galaxy alone has 200 billion stars and most of these stars appear to have planets. The probability of intelligent life somewhere in our galaxy or in another galaxy is high, given these numbers. They compared all existing protein structures that bind metals to establish any common features, based on the premise that these shared features were present in ancestral proteins and were diversified and passed down to create the range of proteins we see today.
They compared all existing protein structures that bind metals to establish any common features, based on the premise that these shared features were present in ancestral proteins and were diversified and passed down to create the range of proteins we see today. That analysis included all cases of COVID-19 confirmed by PCR tests in England in the first half of December in which the variant could be identified: 56,000 cases of omicron and 269,000 cases of delta.
That analysis included all cases of COVID-19 confirmed by PCR tests in England in the first half of December in which the variant could be identified: 56,000 cases of omicron and 269,000 cases of delta. The Food and Drug Administration authorized Pfizer’s drug for adults and children ages 12 and older with a positive COVID-19 test and early symptoms who face the highest risks of hospitalization. That includes older people and those with conditions like obesity and heart disease, though the drug is not recommended for patients with severe kidney or liver problems. Children eligible for the drug must weigh at least 88 pounds (40 kilograms).
The Food and Drug Administration authorized Pfizer’s drug for adults and children ages 12 and older with a positive COVID-19 test and early symptoms who face the highest risks of hospitalization. That includes older people and those with conditions like obesity and heart disease, though the drug is not recommended for patients with severe kidney or liver problems. Children eligible for the drug must weigh at least 88 pounds (40 kilograms).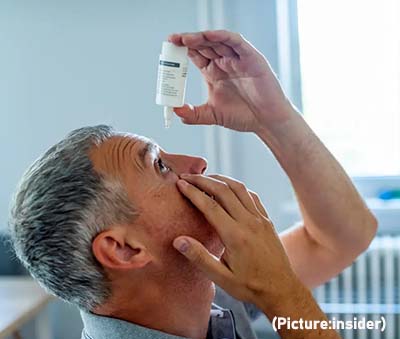 The eye drops will work best for people between the ages of 40 and 55, a Vuity spokesperson told CBS. That age group comprised two clinical trials and is most likely to notice the onset of near vision loss.
The eye drops will work best for people between the ages of 40 and 55, a Vuity spokesperson told CBS. That age group comprised two clinical trials and is most likely to notice the onset of near vision loss.  That makes it the most “massive system around which a planet has been confirmed,”
That makes it the most “massive system around which a planet has been confirmed,”  The new endeavour will base itself a short distance away from the original at Little Dome C, an area located roughly 40km from the Italian-French Concordia Station, on the east Antarctic plateau
The new endeavour will base itself a short distance away from the original at Little Dome C, an area located roughly 40km from the Italian-French Concordia Station, on the east Antarctic plateau “The information people decide to expose themselves to has important consequences for their health, finance and relationships. By better understanding why people choose to get informed, we could develop ways to convince people to educate themselves.”
“The information people decide to expose themselves to has important consequences for their health, finance and relationships. By better understanding why people choose to get informed, we could develop ways to convince people to educate themselves.”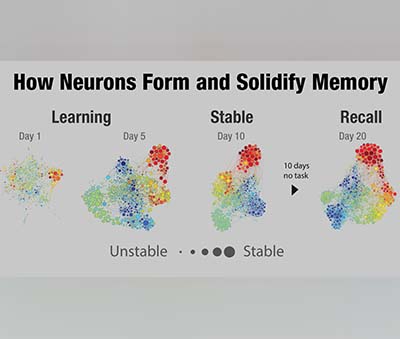 The most significant finding was that firing occurs with different timing relative to other brain activity when memories are being retrieved. This slight difference in timing, called “phase offset,” has not been reported in humans before. Together, these results explain how the brain can “re-experience” an event, but also keep track of whether the memory is something new or something previously encoded.
The most significant finding was that firing occurs with different timing relative to other brain activity when memories are being retrieved. This slight difference in timing, called “phase offset,” has not been reported in humans before. Together, these results explain how the brain can “re-experience” an event, but also keep track of whether the memory is something new or something previously encoded. The variant could lead to severe consequences in some regions, the WHO said on Monday. The head of the organisation, Dr Tedros Adhanom Ghebreyesus, renewed a call for a global push to get vaccines to poorer nations.
The variant could lead to severe consequences in some regions, the WHO said on Monday. The head of the organisation, Dr Tedros Adhanom Ghebreyesus, renewed a call for a global push to get vaccines to poorer nations. Burton said that researchers will know just how effective the vaccines are against this variant “in the next couple of weeks.” If manufacturers need to make an omicron variant-specific vaccine, it should take approximately “two to three months” to test and manufacture it, he said.
Burton said that researchers will know just how effective the vaccines are against this variant “in the next couple of weeks.” If manufacturers need to make an omicron variant-specific vaccine, it should take approximately “two to three months” to test and manufacture it, he said. “From the beginning, we have said that as we seek to defeat the pandemic, it is imperative that we are proactive as the virus evolves,” said Moderna CEO Stéphane Bancel. “The mutations in the Omicron variant are concerning and for several days, we have been moving as fast as possible to execute our strategy to address this variant.”
“From the beginning, we have said that as we seek to defeat the pandemic, it is imperative that we are proactive as the virus evolves,” said Moderna CEO Stéphane Bancel. “The mutations in the Omicron variant are concerning and for several days, we have been moving as fast as possible to execute our strategy to address this variant.” The Ministry also issued an advisory to all ASU Drugs Manufacturers Associations seeking the manufacturers of the crude drug/extracts, sellers, ASU drug manufacturing companies, ASU drug exporters not to use Withania somnifera leaves either in crude or extract or any other form for therapeutic purposes under the ambit of ASU drugs.
The Ministry also issued an advisory to all ASU Drugs Manufacturers Associations seeking the manufacturers of the crude drug/extracts, sellers, ASU drug manufacturing companies, ASU drug exporters not to use Withania somnifera leaves either in crude or extract or any other form for therapeutic purposes under the ambit of ASU drugs. None of this means that Kamo’oalewa has to have especially exotic origins. The solar system is littered with asteroids, some of which are captured by the gravity of other planets and become more conventional—if fragmentary—moons. Others don’t orbit other planets in the common way but fall into line in front of them or behind them and pace them in their orbits around the sun, like the flocks of so-called Trojan asteroids that precede and trail Jupiter.
None of this means that Kamo’oalewa has to have especially exotic origins. The solar system is littered with asteroids, some of which are captured by the gravity of other planets and become more conventional—if fragmentary—moons. Others don’t orbit other planets in the common way but fall into line in front of them or behind them and pace them in their orbits around the sun, like the flocks of so-called Trojan asteroids that precede and trail Jupiter.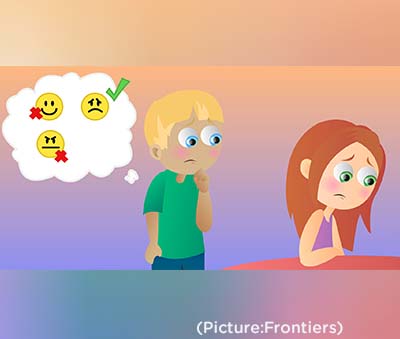 In recent years, researchers have criticised mindreading for being too complicated and demanding to be a common strategy for understanding other people. Julia Wolf provides an example of mindreading: “When I see someone running towards a bus, I infer that this person has the desire to catch the bus,” she says. “In doing so, I can either picture myself in their situation, or draw on my knowledge of general principles regarding the behaviour of others.”
In recent years, researchers have criticised mindreading for being too complicated and demanding to be a common strategy for understanding other people. Julia Wolf provides an example of mindreading: “When I see someone running towards a bus, I infer that this person has the desire to catch the bus,” she says. “In doing so, I can either picture myself in their situation, or draw on my knowledge of general principles regarding the behaviour of others.”