In late May, The US Food and Drug Administration (FDA) allowed for Emergency Use Authorization (EUA) to combat the COVID-19 pandemic, resulting in the investigational monoclonal antibody therapy sotrovimab. This new therapeutic weapon allows for the treatment of mild-to-moderate COVID-19 in patients over 12 years of age with positive outcomes. Its widespread use includes patients who are at a higher risk of more severe symptoms of COVID-19 such as individuals who are over 65 years old or those with certain medical conditions.
With a lead over the first-generation monoclonal antibody (mAb) therapies for COVID-19, Sotrovimab is reportedly referred to as super-antibodies due to their broad neutralization capacity when encountered with viral pathogen variants.
Sequencing each version of the virus the patients are suffering from would not only be overly meticulous but also equally painstaking. Therefore, Sotrovimab’s large range in capabilities is enticing to physicians as stated by analysts and researchers.
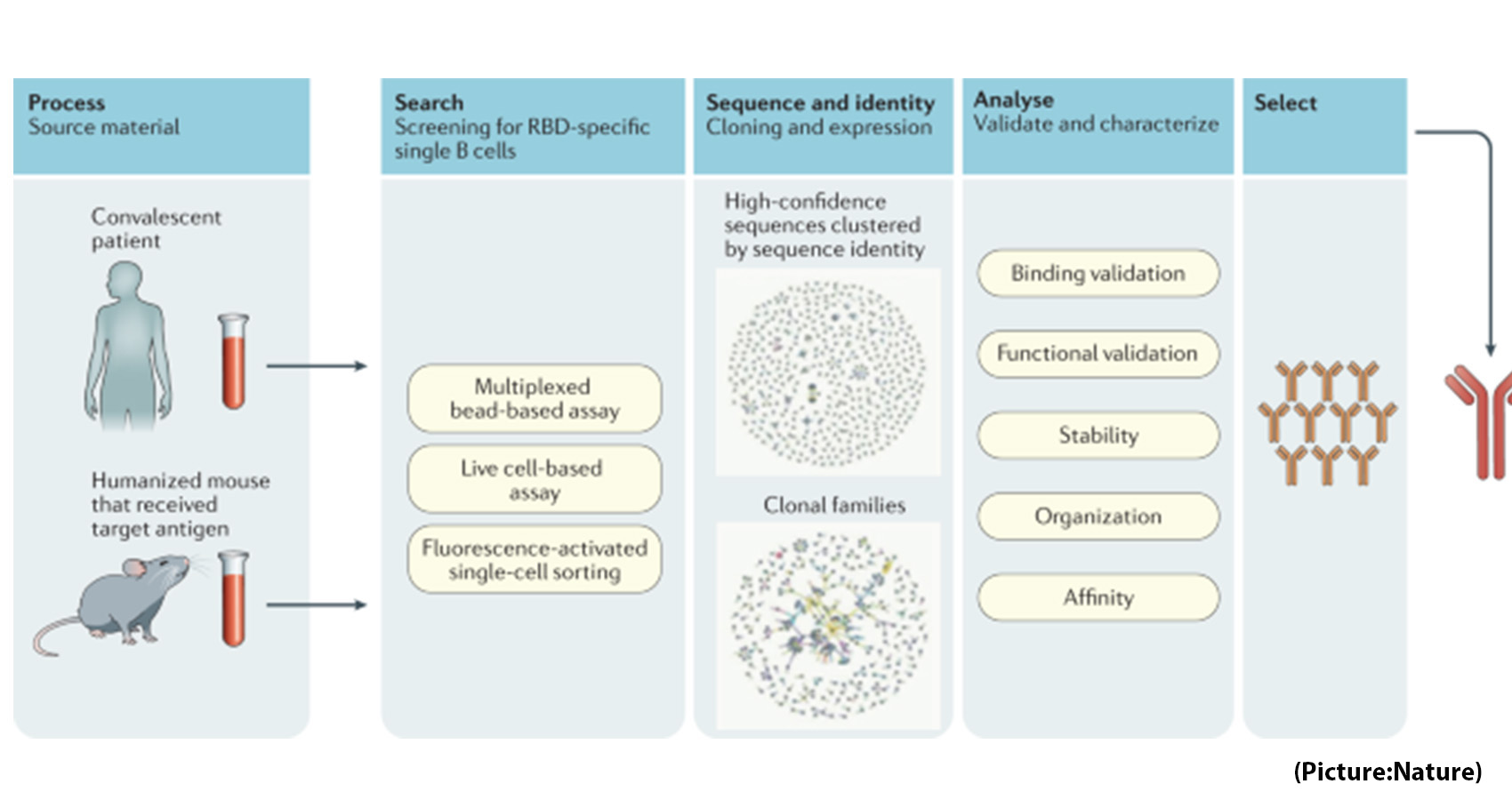
 When a person is infected with COVID-19, antibodies are typically produced to fight against the invading disease. These antibodies are unique to each individual, meaning that some antibodies are better than others at combating the virus. Thus, pharmaceutical companies study thousands of these antibodies to take advantage of the most effective ones with the highest barrier to resistance.
When a person is infected with COVID-19, antibodies are typically produced to fight against the invading disease. These antibodies are unique to each individual, meaning that some antibodies are better than others at combating the virus. Thus, pharmaceutical companies study thousands of these antibodies to take advantage of the most effective ones with the highest barrier to resistance.
Out of these pharmaceutical companies, Vir Biotechnology and GlaxoSmithKline derived the monoclonal antibody sotrovimab from patients who had SARS in 2003, which targets parts of the COVID-19 virus that it shares with the original SARS virus. By targeting these areas, it lowers the chance for the virus to mutate and should allow for the antibody to work against new variants.
According to data from clinical trials, sotrovimab showed positive results with a 79% reduction in risk of hospitalization or death, appearing to yield activity against the currently known problematic variants of COVID-19.
Sales for these antibody therapies were expected to diminish as vaccination rates continuously rose, however, analysts predict that the market for COVID-19 mAbs will endure to assist in treatments for those who are unable to receive their vaccination shots for medical reasons.