In a groundbreaking discovery, researchers led by Ali Shilatifard, Ph.D., the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics, have identified a novel repeat gene cluster sequence exclusively expressed in both humans and non-human primates. This significant finding, outlined in a study featured in Science Advances, marks a pivotal moment in human genome biology, offering far-reaching implications for future investigations into transcriptional regulation, human evolution, and the exploration of repetitive DNA sequences.
Shilatifard, also the director of the Simpson Querrey Institute for Epigenetics and a professor of Pediatrics, expressed the magnitude of this breakthrough, stating, “This is an unbelievable discovery of the first elongation factor that is repeated within the human genome and is very primate-specific.”
The evolution of genome sequencing technologies over the past two decades has facilitated in-depth exploration of the genetic landscape within various regions of the human genome. Previously elusive large sections, often composed of repetitive DNA sequences referred to as genetic “dark matter,” have become identifiable through recent advancements in long-read DNA sequencing.
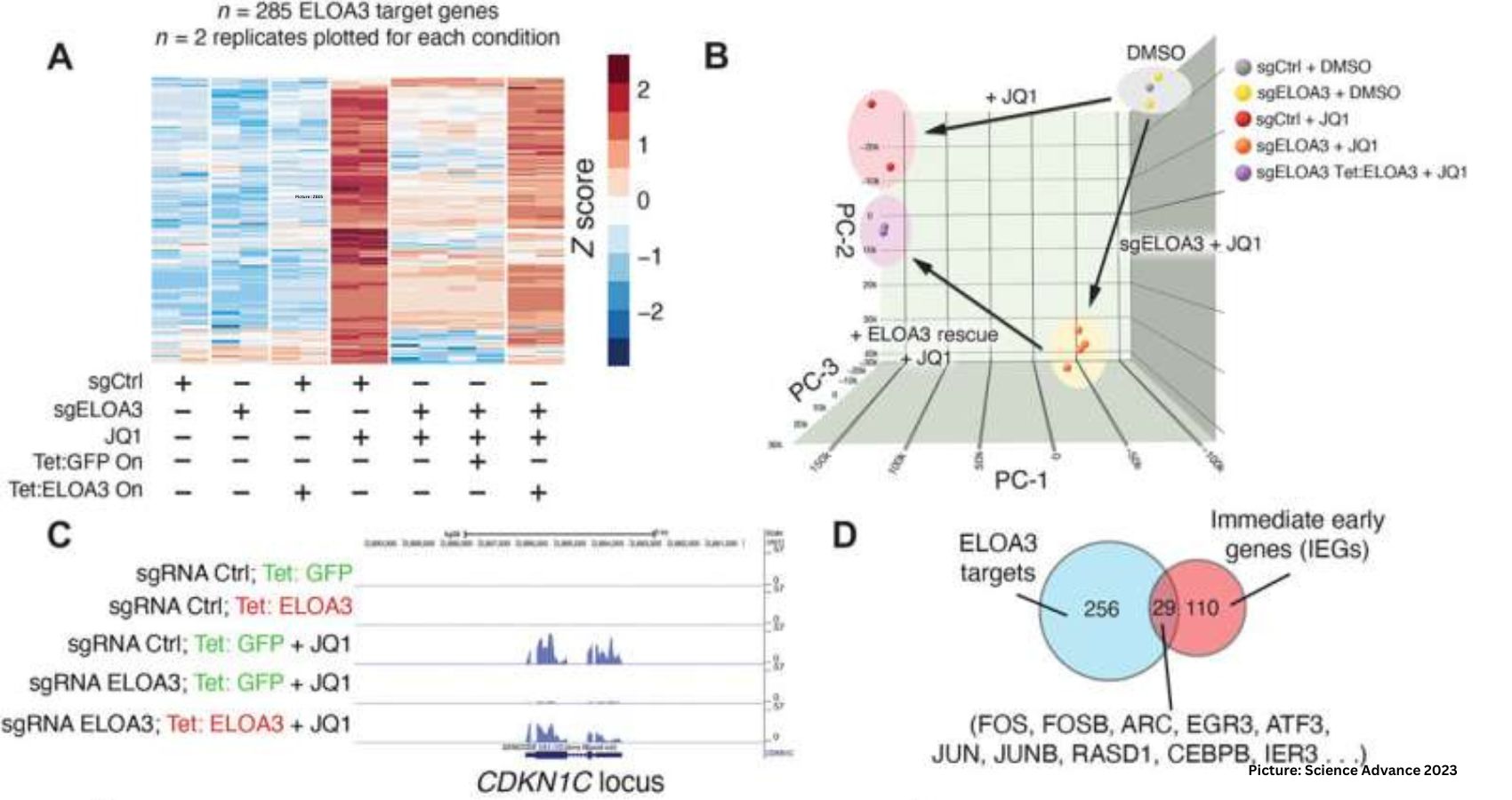
During the investigation of a cancer-inhibitor compound in human cell lines, Shilatifard’s team serendipitously uncovered a cluster of previously uncharacterized genes responsible for encoding the Elongin A3 (ELOA3) protein. This protein, closely linked to the previously studied ELOA protein, plays a crucial role in regulating RNA polymerase II (RNAPII) transcription, a fundamental process in gene expression.
Marc Morgan, Ph.D., former Associate Research Professor in Shilatifard’s laboratory and co-lead author of the study, emphasized the uniqueness of the ELOA3 gene cluster. Unlike the conventional scenario where a single human protein is encoded by a single gene, the ELOA3 cluster features multiple genes located in the same genetic locus encoding identical proteins, presenting an intriguing subject for investigation.
Collaborating with Evan Eichler, Ph.D., professor of Genome Sciences at the University of Washington School of Medicine, the researchers found that the ELOA3 gene cluster is exclusive to humans and non-human primates. Furthermore, the number of ELOA3 gene repeats varies among individuals and across primate species, suggesting a dynamic element in its evolutionary history.
Shilatifard highlighted the variability in repeat units among individuals, stating, “You may have 52 copies, I may have 27 copies, somebody else may have 32 copies. We don’t know why, but we know that there are different repeat units in every individual, and this repeat unit is highly conserved among primates.”
The research team proposed that the ELOA3 gene cluster has undergone concerted evolution and gene homogenization within the examined primate species, a conclusion drawn from observations of its consistency among primates.
Utilizing protein biochemistry techniques, the researchers demonstrated that ELOA3 forms a distinct protein complex from the ELOA protein, employing unique biochemical mechanisms to regulate RNAPII transcription. This discovery not only advances our understanding of human genome biology but also provides insights that could lead to targeted drug design in cancer.
Saeid Mohammad Parast, Ph.D., a postdoctoral fellow in the Shilatifard laboratory and co-lead author, emphasized the significance of ELOA3 in understanding gene expression variability, stating, “The dynamic nature of the ELOA3 repeat cluster could be a reflection of its unique role in regulating gene expression variability among individuals.”
The researchers’ future endeavors include determining the expression patterns of ELOA3 in the genomes of humans and non-human primates, exploring its potential role in developmental disorders and diseases, including cancer. Morgan emphasized the evolutionary conservation of this gene cluster among primates, suggesting it could unveil unique gene regulatory mechanisms specific to the human evolutionary lineage.